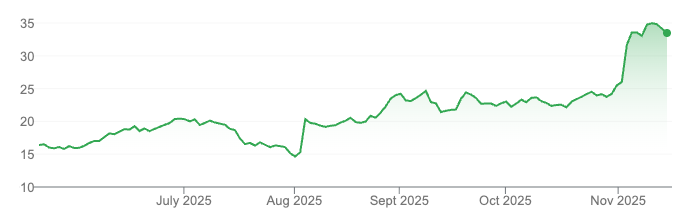
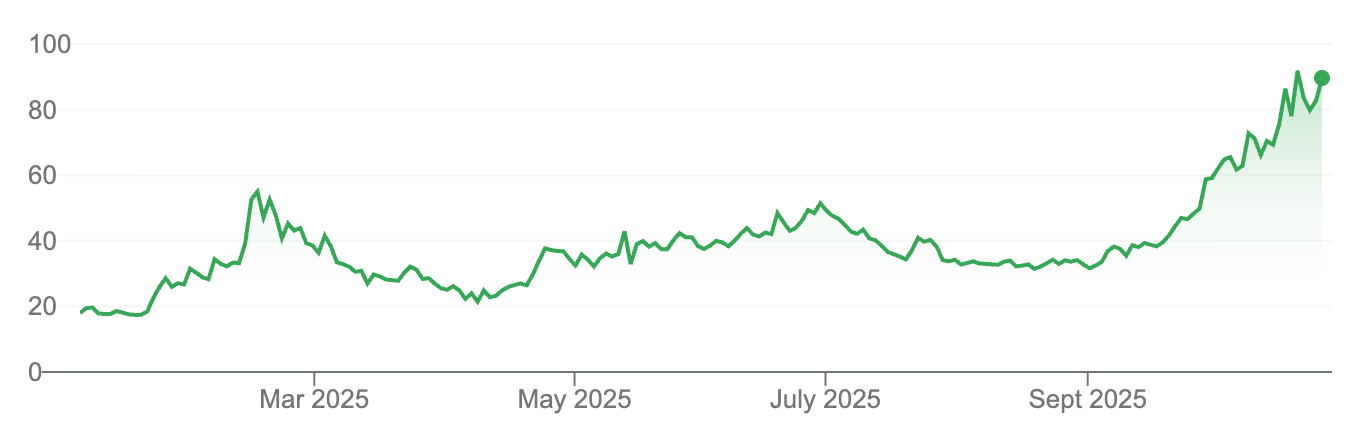
It has been a long 1,000 day bear market for biotech investors.

Since June, medical technology and major pharmaceutical companies finally joined the sell-off.
If you knew that Pfizer would develop a vaccine for a global pandemic that would become mandatory around the world, how do you think that stock would trade? Probably not like this!

A thousand days ago CRISPR companies were valued in the tens of billions of dollars with early stage pipelines and limited clinical data, if any at all, and were mostly focused on the same small set of blood diseases and cancers. Competition in biotech is a killer, as it means companies can lose big, even if they win regulatory approval, which is hard enough! Runners up have to prove they work and are better than the incumbent, which has more data and established clinical practice.
Some of the most expensive companies in 2021 sold advanced scientific equipment to academic institutions and corporate research labs, which are budget-constrained customers at the best of times.
Even now, after a 90% fall, it’s not clear how to value (to give one example) Pacific Biosciences, when it’s losing $309 million on $170 million of revenue. The current $1.7 billion market cap might actually be too high.

There are, however, new developments in the last week or so that suggest a turn might be close.
- After a positive FDA advisory committee meeting, the first CRISPR treatment is on the cusp of approval
- Verve Therapeutics released the first in-human base editing data, which worked (with caveats)
- Eli Lilly announced a major investment in early stage biotech companies
- Detail from Novo’s SELECT Ozempic trial suggests the drug is not going to cure all heart disease
- Finally, it’s about time
After years of promise we are at the cusp of the first CRISPR approval
An FDA advisory committee met to discuss CRISPR Therapeutics lead candidate in sickle cell disease.
The questioning centered around whether the firm had sufficiently measured the risk of off-target effects, where gene therapies mess about with the wrong part of the genome.
The studies were small, but the benefit to very sick patients was clear. A general consensus has formed that the treatment will be approved.
This would mark the first CRISPR approval after years of hype, billions of investment, and billions more wagered and lost.
1,000 days ago these companies had very little hard clinical data to show and were priced as though their current and future pipelines were all going to work. Today, the situation is the opposite. We have three more years of data, and many of these companies are priced to fail.
Eli Lilly invests!
Eli Lilly announced it was buying Beam’s partnership rights to Verve Therapeutics base editing programs for $200 million cash and up to $350 million in milestone payments.
Lilly also made a $50 million purchase of Beam stock at above the pre-announcement share price. Beam has $1.07 billion of cash and a $1.9 billion market cap, so this is significant.
Beam specializes in next-generation base editing technology, while Verve is applying that technology to heart disease - an unusually ambitious target.
This offers the tantalizing prospect that Eli Lilly will use some of its sweet GLP-1 cash to fund the advanced technology sitting in massively oversold biotechs.
With a partner like Lilly, these programs will be funded to completion, provided the data holds, of course.
Right on time, Verve released data proving that base editing does work in humans
Verve released the first data showing base-editing works in humans, where a single base pair is corrected, rather than the blunter cuts and edits of CRISPR.
This is an excellent proof-of-concept for the next generation of gene editing companies. But there was at least one heart attack and maybe two which could be trial related. This is a problem in such small trials as it’s particularly hard to tease out cause-and-effect, so it’s a good thing Lilly is there.
As befits a Phase 1 study, these were extremely ill patients with heart disease no less. Teasing apart cause and effect here will be difficult.
The good news for Beam is that they sold their rights to these programs and the proof-of-concept works. And for Verve, given Lilly’s immense resources, there will be further funding, which was not a given only two weeks ago.
Novo Nordisk released detailed data on their study testing semaglutide’s effect on heart health
A few weeks ago Novo released the headline results, which showed semaglutide did reduce the risk of cardiovascular events, triggering a staggering sell-off in all kinds of usually stable medtech companies, which benefit from an aging, fattening population.
But the detail looks less impressive than the headline.
Firstly, only 132 serious cardiovascular events were avoided in a patient population of ~8000 over a number of years. Specifically, there were 701 cardiovascular events in the placebo group, and 569 in the semaglutide group. This is a solid win, but the population was already old, overweight, and in poor health. So no panacea.
More importantly, the cost-benefit does not quite stack up. 132 cardiovascular events were saved, but patients on stayed on the drug for 35 months on average. At a ~US$1,000/month cost, this represents millions of dollars per missed heart attack. At the margin this helps justify the cost and is a step forward in the right direction for heart health, but it is not a revolution. GLP-1 agonists may be revolutionary for weight loss, but so far it seems the impact on heart health will be modest, and in-line with what you’d expect from weight loss alone.

There were two other interesting data points. Firstly, the patients only lost ~9% of their bodyweight, which is less than in other trials.
Secondly, while the vast majority of health indicators trended in the right direction, there was one standout: heart rate increased.
It’s the season
Finally, in terms of sentiment and valuation, we are breaching lows reached in 2022, with more companies trading for less than cash than ever before.
This doesn’t augur a turn necessarily, but it does suggest where we are in the broader timeline. If you want to invest in Winter and harvest in Summer, it’s clear where we are today. There is certainly little ‘upside’ priced into companies trading for less than cash, and the rallies after similar bear markets, such as in the early 1990s, were spectacular and lasted the rest of the decade.
The 1,000 days since the top in early 2021 have been a long, drawn-out strangle. A record number of companies traded below cash first in 2022, then after a brief rally again in recent weeks when this new record was exceeded.
But seemingly all at once we have the first approval in CRISPR coming up, an (up-to) $600 million cash injection by Eli Lilly into early stage biotechs, which hopefully marks the beginning of the recycling of immense GLP-1 revenues into early stage companies, and data is finally starting to show the limits of GLP-1 drugs, which have cast such a pallor over the healthcare space. Time will tell.
If you'd like to invest with us, you can access our investment portal and fund documentation through the button above.
Or simply reply to this email and we'll be in touch.
Disclaimer
The information in this note has been prepared and issued by Frazis Capital Partners Pty Ltd ABN 16 625 521 986 as a corporate authorised representative (CAR No. 1263393) of Frazis Capital Management Pty Ltd ABN 91 638 965 910 AFSL 521445. The Frazis Fund is open to wholesale investors only, as defined in the Corporations Act 2001 (Cth). The Company is not authorised to provide financial product advice to retail clients and information provided does not constitute financial product advice to retail clients.
The information provided is for general information purposes only, and does not take into account the personal circumstances or needs of investors. The Company and its directors or employees or associates will use their endeavours to ensure that the information is accurate as at the time of its publication. Notwithstanding this, the Company excludes any representation or warranty as to the accuracy, reliability, or completeness of the information contained on the company website and published documents.
The past results of the Company’s investment strategy do not necessarily guarantee the future performance or profitability of any investment strategies devised or suggested by the Company.
The Company, and its directors or employees or associates, do not guarantee the performance of any financial product or investment decision made in reliance of any material in this document. The Company does not accept any loss or liability which may be suffered by a reader of this document.