Hello all,
I recorded a conversation with Paul Anderson, CEO Orthocell (Spotify, Apple). We invested in their raise at 60c and it’s now a 5% position in our main fund.
As a follow up to Syntara, this deal was just announced two weeks ago for what we see as an inferior product at a similar stage of development.
A$300m upfront, ~3x Syntara’s market cap, with royalties which could be substantial.
Orthocell transcript below.

Michael Frazis
I’ll just throw you the ball. What does Orthocell do and what are your plans for the future?
Paul Anderson, Orthocell CEO
We're a medical device company that works in the regenerative medicine space.
We've developed a collagen-based platform which consists of three main products:
The first product was for human bone regeneration.
The second is for regeneration and repair of nerves in the upper limb predominantly, but also all over the body.
The third is for tendon repair.
We work in what's called the musculoskeletal space.
We have a collagen platform in the medical device space that has evolved three products that are either in market or moving to market.
Michael Frazis
Great, let’s start with your bone regeneration product.
How does it work? Who are your customers?
Paul Anderson, Orthocell CEO
The bone was the first product we took to market.
It’s now approved in Australia, the US, Europe, Canada and soon in other jurisdictions, with applications underway in Brazil, Colombia and Singapore.
It’s predominantly used by dentists in what's called maxillofacial surgery or surgeries of the jaw. Our product augments bone growth in the jaw where bone reabsorption occurs.
This is centered around the use of dental implants, as 40% of cases lack sufficient bone.
To get that successful implant, you need a bone graft. Each bone graft needs to have a barrier membrane, or a collagen membrane that sits over the top. And that collagen membrane protects it from the outside world.
Our product does two things. It protects the bone graft from the outside world, but it also encourages the bone to repair in a faster and more predictable fashion.
It's used in conjunction with bone graft and it's a product that enables dental implantation.
This is one of the fastest growing areas of dental surgery today.
We're partnered with BioHorizons, 66% owned by Henry Schein, one of the largest dental companies in the world.
BioHorizons paid us $21 million net of fees upfront for an exclusive license and distribution agreement.
Our product enhances their dental implant success and market reach.
Our relationship paid exclusivity with BioHorizons has allowed us to access global markets. We manufacture the product and sell it to them for distribution.
Michael Frazis
What's the revenue of that business and what do you think the opportunity is globally?
Paul Anderson, Orthocell CEO
Globally this market is $800 million to $1 billion. It's one of the fastest growing areas in dentistry.
We've just ticked over close to a million dollars worth of revenue. We're really excited about our partner and their expertise in the space.
They own more than 20% of the market globally, which provides us with a fantastic opportunity and the ability to tap into that $800 million market.
Michael Frazis
That market sizing of $800m to a billion, is that specifically for what your product could be used for?
Paul Anderson, Orthocell CEO
Specifically for our products.
The bone regeneration product is a lower margin, higher volume product, compared to our nerve product, which is high volume and high margin.
Our relationship with Bio Horizons has attracted non-dilutive capital and opened pathways to international markets, validating our manufacturing profile.
We monetized that opportunity, validated by the due diligence done by BioHorizons and Henry Schein on our product, innovation, intellectual property and manufacturing capability.
It also allowed us to scale our manufacturing operations, for our bone product and the nerve and tendon products to follow.
Michael Frazis
What would it take to increase from say $1 million revenue to a more substantial part of that market? What do you think will determine that?
Paul Anderson, Orthocell CEO
We've just scratched the surface.
Our product has been with BioHorizons for a couple of years, successfully launched in the US, and expanding internationally to Austria, Switzerland and Germany.
Growth will come from BioHorizons as they request additional jurisdictional applications. We will see significant growth potential in international expansion.
Michael Frazis
Let’s move on to Nerves and Remplir.
What is the product and how do you make it?
Paul Anderson, Orthocell CEO
Remplir is from our collagen platform.
A unique product differentiated from anything else on the marketplace.
It’s placed into the nerve tissue to join two nerves together, that have been severed or crushed, and have caused paralysis in limbs.
The traditional method is to join the nerves using a needle and thread, first publicised back in the 1860s.
Effectively, we're still doing the same thing now. However, we've become more sophisticated in how we apply those needles.
The application of the needles causes scar tissue in the nerve itself. No matter how good a surgeon you are, some degree of scar tissue forms.
This inhibits a consistent and predictable growth of the nerves.
Remplir can join nerves together by either reducing or negating the use of the damaging sutures. As an adhesive, it sticks the nerves together.
It also provides a bioactive chamber, which enhances the nerve's ability to heal.
It reduces scarring and enables a consistent and predictable return to function to paralyzed limbs.
An exciting product in a large global market segment.
Michael Frazis
Pretty incredible to use needles and thread on a nerve. How do you even use that on something that's quite small and narrow?
Paul Anderson, Orthocell CEO
You'd be surprised at the size of some nerves in our body, they vary from microscopic to the size of your little finger.
Which is the point; how do you control a needle in a zone like that?
We've done usability studies with some of the best surgeons in the world, it's almost impossible not to prang the nerve when doing a repair.
Our product reduces surgical time to repair these nerves by reducing the number of sutures needed and creating a better environment for healing. Allowing for less nerve handling and risk of damage.
We're reducing overall surgery time, which is very good for the patient, and good economically for the healthcare community.
Michael Frazis
If we look at the images in your presentations, it looks like a piece of paper that wraps around the nerve?
Paul Anderson, Orthocell CEO
Great question. The product is a soft, compliant piece of tissue designed to mimic human collagen, and the outside edge of the nerve (the epineurium).
It's implanted and helps join the nerves together, then degrades and integrates into the nerve itself, ultimately mimicking the tissue already there.
Michael Frazis
Now in terms of alternatives, what are the other options surgeons might have?
Paul Anderson, Orthocell CEO
We're not absolute pioneers in this space. We didn't invent the use of collagen to repair nerves.
What we did invent was a very unique product in the domain.
Competitively, we see the likes of Axogen and Integra Lifesciences, generation one products.
They're stiff, hard conduits that take a long time to absorb water and become compliant. They cause scar tissue and don't degrade well within the body.
We've seen it validated by those companies. But there's also now a realisation that it's not the best design and not fit for purpose.
We've learnt from their experiences and have developed a product fit for purpose.
Our product has the capability to integrate and degrade at the right time, which other products don't do. They either last too long in the body and cause inflammation or they don't last long enough and are eaten up.
One of the great advantages of this product is that you can feel it and you can touch it and you can see the benefits of it right there in your own hands.
As soon as you put it into a surgeon's hands, they stop talking and are enamoured by its compliance.
Michael Frazis
How is it manufactured? Can you go into some detail on that?
Paul Anderson, Orthocell CEO
We have a series of ISO accreditations here at our facility in Australia.
We're very proud manufacturers of the product and have developed a strong and robust system. It has been approved by multiple international jurisdictions.
We bring in raw animal material and chemically and mechanically manipulate it.
We take a virtually valueless piece of animal material and turn it into an extremely high value medicinal product.
We now manufacture that for local patients and international partners.
Michael Frazis
What kind of trials did you do to get this to market? Did you have to go head-to-head?
Is there benefit in outcomes, as well as surgeon convenience?
Paul Anderson, Orthocell CEO
We have generated clinical data and we've recently published our initial clinical paper in Journal of Reconstructive Microsurgery.
What we showed in that paper was an 85% success rate in paralysed limbs.
We were able to take patients who had nerve damage which either paralysed their hands, arms or whole upper trunk and showed a consistent and predictable clinical outcome.
The surgeons started with basic nerves in the hand and the fingers. Then moved to more complex nerves in the shoulder and the elbow. As confidence grew, we pioneered nerve transfer.
Taking nerves from other parts of the body and transferring them into areas that are paralysed.
For example, we now treat and have done a lot of work with quadriplegic patients.
Patients that have had a catastrophic neck injury, causing paralysis of their body, but who can still breath for themselves.
Meaning there are still nerves they can control in their chest and thorax area.
We take redundant nerves from their chest and re-plug them into their triceps and biceps.
Quite phenomenal outcomes. We have a number of case studies.
An Australian champion downhill cyclist, a mountain biker, fractured his neck.
Nine months after his injury, he had our surgery. Two years and two months later, he drove his own car down to visit our facility.
He had incredible mobility return. He could use his arms, power his wheelchair.
He said to me, Paul, the most important things that you have done for me is that you've given me the ability to have access to the outside world via my phone.
He said, you have no idea about the ability for me to pick up a phone, turn it over and use my fingers to dial somebody and talk to them on that phone.
He said, to be able to drive my vehicle, to get a job, to have self-esteem, to be mentally healthy and strong.
What you have done is given me the ability to hug my children and hug my wife, to have physical connection, to be able to touch and feel.
It is a thing that we take for granted. And when you see patients that don't have that ability to have the return of their mobility, the return of their mental health, the return of the ability to hold their loved ones has been a truly fantastic moment for Orthocell.
But most importantly, it's been a fantastic moment for our patients.
We have a product here that is making a really big impact on patients, on surgeons, and presenting an incredibly strong cost economic developmental piece for the healthcare industry.
Our website (link) has several videos of young ladies and men who have been terribly compromised. And we're proud that we've been able to change people's lives.
The delivery of this technology into the Australian marketplace is nothing short of phenomenal.
I've been involved in product launches in the US, Europe and Australia.
The product launch of Remplir has been used by over 161 surgeons and is used in over 130 hospitals across the country in the last 18 months.
To have that kind surgical uptake between plastics, reconstructive surgeons and orthopaedic surgeons has been fantastic.
Michael Frazis
Coming back to commercials, in that example of somebody who's quadriplegic, how is the product used?
Is your product wrapping around the nerves so they reconnect?
Paul Anderson, Orthocell CEO
Correct.
You have to cut the live nerve, then thread that into the damaged nerve to join them together.
Think of an electrician, essentially taking a live wire to a dead wire and plugging them together to innovate the dead wire. But unlike electricity, it takes time.
It grows by about a millimeter a day. We provide a pathway for that live nerve, which happens over time.
Michael Frazis
Presumably it takes time and training for the nerves to reorganize and be controlled again?
Paul Anderson, Orthocell CEO
It does. There's three really important elements here.
Let's have an outstanding product that's fit for purpose, to have surgeons that can do these operations.
There is a rehabilitation quotient tool with occupational therapists who motivate the patients to continue to reconnect their brain function to their injury.
A result of the clinical study is that a large number of these patients that we treat are all on opioid or other very heavy painkilling drugs.
After Remplir, we were able to reduce the use of narcotic analgesics to almost zero.
A big cost economic productivity benefit to the patients and the healthcare community.
Michael Frazis
So my understanding is it is approved in Australia, but not in United States, which we’ll come back to.
What incentivizes people to choose your product over another, you able to shed some light on the competition as well?
Paul Anderson, Orthocell CEO
Orthocell has developed what we believe is an unparalleled scientific narrative.
The construct we’re developed in the shape of the product, its unique characteristics, and the ability for it to reabsorb and integrate into the body.
What we have is a product different from others in the market, fit for purpose.
Our background in regenerative medicine means we approach this from a cellular level.
Most of the other companies in this business approach it from a medical device perspective, a strengths perspective.
Strength is important, but it's not everything.
Your product needs to integrate with the tissue and degrade at the right point in time.
Our product has learned from these Generation One products. It is unique and provides us with consistent and predictable clinical outcomes, which other companies struggled to do.
Michael Frazis
In one of your presentations, you stated only 10% of people used one of your competitors.
Why is that? What are they using instead?
Paul Anderson, Orthocell CEO
The market leader today is a company called Axogen. They hold less than 20% market share, demonstrating that there's been strong validation in the approach of using collagen medical devices to reduce or negate sutures.
Most of the market uses sutures. Our target is not necessarily our competitor, our target is sutures.
We're demonstrating a great scientific narrative and good clinical data to support that, that we can reduce suture time and it's easier for the doctor to use. On all parameters, we're making a better outcome for the doctor and the patient.
Axogen is the market leader, but they don't make their own product.
Integra makes their own product with 20-year-old technology.
The two market leaders have struggled to gain further market share because their products aren't quite right. We’ve seen that the market is validated, surgeons want alternatives, and we have the perfect product.
That's the pathway we're on now into the US.
Michael Frazis
Where are you at in terms of the US approval and launch?
Paul Anderson, Orthocell CEO
We are going down a 510k pathway, which is important to entry to the US. We have recently articulated the success of the animal study that we're doing to the market.
This pathway needs animal data to get access to the US marketplace and you compare against another product.
Our successful animal data has now been rolled into a submission. We'll be making an FDA submission for clearance by the end of Q4 2024.
We're in the final stages of preparation for that application. A 510k application has a legislative 90 days, we’re hopeful we can gain approval in Q1 25.
We've got a really strong program. We've worked with the FDA with two pre-sub meetings and are confident in our pathway to gaining access to that really lucrative and exciting US marketplace.
Michael Frazis
And it is already approved in Australia and elsewhere, so US approval looks likely.
Paul Anderson, Orthocell CEO
Across the whole portfolio of products, we've implanted in over 60,000 patients now.
This demonstrates the uptake that we’ve had across the platform. It is incredibly safe.
We’ve gained approval across many jurisdictions, our platform has products in Australia and New Zealand, Singapore, US, Canada, Europe and the UK. It’s been validated by global regulatory authorities on numerous occasions.
The risk is very low, we have a robust package and have been validated by great consultants with strong track records in high quality regulatory submissions.
Michael Frazis
One problem with the 510K pathway, which is different to say small molecule drugs, is that the bar is lower. So it's easy to get approved, but often not definitively proven to be better than the standard of care.
There's competitors in the market so success can be more about sales strategy and marketing.
What's the marketing plan? How much are you planning on spending? Can you talk about your strategy to actually get it to market?
Paul Anderson, Orthocell CEO
So you need to have good clinical data. You can't sell a piece of popcorn without a bit of data.
We have that in two elements. We have that in published clinical data. And we also have real world evidence, which is post-market clinical follow-up data.
This is a strong data package for the surgeons, underpinned by strong science.
The science is multiple animal studies. In the nerve space, animal studies are an important part of building knowledge and evidence to use the product.
Your next comment, which was very relevant, was how do you get it to market? And what's the strategy there?
We've been in a fortunate position for Striate, we were able to get that product into the European and US marketplaces.
In Australian marketplaces, we have clinical traction. We had a team of 16 key opinion leaders as our advocates and our product champions.
You need to have a product that's appropriately priced, that has the right champions in the marketplace and is distributed across a broad range of hospitals and jurisdictions.
We’re going to invoke a hybrid model. So we won't be spending huge amounts of capital on direct workforce. We think it can be done equally as well through the multitude of networks of distribution partners that exist in the US. The world's largest healthcare market and largest number of healthcare distributors.
We will work with our senior management as direct sales, our product managers, and distributors within that.
Following our initial introduction, we will work alongside our key opinion leaders such as Massachusetts General in Boston and Washington University in St. Louis.
We have been working on these key opinion leaders and developing those networks for the past two years. We have been successful and leads us with great confidence to gain market traction in the US.
Michael Frazis
What are the key selling points?
Do you have data that shows this is a better outcome than sutures? What are you actually putting into the package?
Paul Anderson, Orthocell CEO
Creating a package for the surgeon, you're creating trust and faith for that doctor through the pathway.
The bench work, multiple animal studies, clinical work and post-market clinical work create our full package. This gives confidence to the surgeons using our products.
When I tell surgeons that it's been implanted into about 60,000 patients now, that gives them comfort that we have a safe and efficacious product.
Michael Frazis
What was the thinking behind launching outside the United States first?
Paul Anderson, Orthocell CEO
This is something I get asked a lot.
I have spoken with over 20 key opinion leaders out of the US, and everyone has asked if we are approved in our own market first and if we have clinical data.
They want to know we’re you're not coming to the US because it's easier to get into. In Australia and Singapore you need clinical data to be approved and in the US you don’t.
The clinical data that we've generated provides us with a really strong evidence base to move into that market. The strategy was to get approved in our own jurisdiction first and to get reimbursement.
We can now demonstrate to global partners, investors, distribution and potential acquirers down the track, that we have a reimbursable product.
A strong strategic decision, reimbursement, regulatory approvals, knowledge gain, and then move all of that into the US marketplace.
Michael Frazis
Let’s talk about margins.
What are they for Remplir now and what are you targeting?
Paul Anderson, Orthocell CEO
Remplir has a 90% gross margin.
Across the product portfolio, it's about a 75% to 80% margin as Striate is lower. These margins provide us with a really good business model.
We've assessed this as about a $3.5 billion market internationally with Australia, US, Europe, Singapore, Canada, Brazil and Japan.
For us to conservatively attack 20% of that market share, it's realistic that we reach $355 million in revenue.
We have the package, product and clinicians to drive it.
It is a really exciting business opportunity for us and we’re only scratching the surface. There are more jurisdictions to explore.
Remplir is also used to protect nerves. It can be wrapped around the nerve in amputations, like a chef’s hat, to aid the placement of prosthetics to avoid painful neuromas.
So not only is there a market of $3.5 billion, but there is also organic growth around it.
Michael Frazis
Let's zoom back in on market sizing.
What's the actual price of a square sheet of this?
Paul Anderson, Orthocell CEO
We’re reimbursed $1,380 for a 1.5 by 2 centimeter square piece of this in the Australian marketplace. Total surgery costs can be up to $15,000.
The addressable market there is large. The cost economics is strong and the cost of our product individually is not off the Richter scale.
We are price competitive. We have a higher quality product than our competitors but we want to attract surgeons and hospitals.
It is $2,500 for the largest sheets, which is really competitive pricing from a healthcare perspective.
Our Chairman John Van Der Wielen has been strong in articulating this story.
As the ex-CEO of HBF, one of Australia's largest healthcare insurers, he was particularly enamoured by this product because of its positive cost economic benefits for hospitals and health insurers.
This has a strong clinical benefit and a strong economic benefit to society.
Michael Frazis
How different is it from that competitor that you mentioned?
Paul Anderson, Orthocell CEO
It's very different.
In handling qualities, theirs are stiff and rigid, right? You have to soak them in water between 20 and 40 minutes. Ours, you dip in water and it absorbs instantaneously so there’s no delay in the surgery time.
From an inventory perspective, we have three main sizes in our product range. Our competitors have up to 25 sizes because their product has to fit the nerve. Hospitals love it because they don't have the large inventories
We're different in feel. Certainly different in the origin of the tissue as the competitive products come from intestinal tissue. We now know from a medical perspective that intestinal tissue is difficult to manufacture and has a greater inflammation risk.
Michael Frazis
Do you think you're going to need a sales distribution partner the same way you did with Striate?
Paul Anderson, Orthocell CEO
Our initial market entry will be with smaller distributors.
Even the largest companies in the US, such as the Strykers and the Biomeds of the world, still use distributors as well as a direct sales force.
Our plan to attract a key partner, is for the next 18 months in the US, to grow market traction, the number of hospitals and appreciation from the hospital and investment communities.
Key opinion, advocacy, senses of excellence, growing revenues, and a differentiated scientific narrative.
They're the four things, and we have every single one of those.
Michael Frazis
It's a $250 million market cap?
Paul Anderson, Orthocell CEO
Yes.
Michael Frazis
If you get near $350 million in revenue, that's an enormous return potential for the stock.
Do you have the financial capacity to launch this product after your raise?
Paul Anderson, Orthocell CEO
From a company perspective we are in a wonderful position.
We recently raised $17 million to turbocharge our scenarios into the US, which provides us with about $33 million.
We are extremely well capitalised, we have the product and capacity to deploy.
We're going to have it registered and ready to go with key opinion leaders ready there.
We've recently refashioned our board.
Professor Fiona Wood, Australian of the Year, a great entrepreneur, inventor, she's had great successes, some failures, which I appreciate as much as the victories.
Kim Beazley is on the board, one of the most bipartisan ex-politicians. Incredible talent, an ex-road scholar, ex-governor of Western Australia, ex-ambassador of the US, and has deep defence knowledge and connections in the US marketplace. He's going to help us with our defence grants and moving into that space, specifically in the capacity of treating bullet wounds.
Ravi Thetani, CEO of Emory Hospital Group in Atlanta, ex-director of research at Mass General and Harvard University. He's an international healthcare heavyweight.
John Van Der Wielen brings global financial expertise as the ex-CEO of HBF for five years, he knows the healthcare industry well. Deep financial services background; having led large companies in Europe and the UK.
We’ve fashioned our board for our next phase. We're really proud that this board can drive us into the future.
Michael Frazis
Great.
Before we wrap up, can you give us an update on what to expect from the calendar of the next 12 months?
Paul Anderson, Orthocell CEO
We'll see the US submission in Q4 of this year.
We're aiming to have FDA clearance and the product approved by the end of Q1 2025, a really exciting milestone on our doorstep.
In the first half of 2025 we will be submitting Remplir for Canada and the UK. Continued regulatory approval and regulatory submissions. Asian expansion in Thailand, the Philippines and Vietnam.
We're excited about clinical data that's going to be released along the pathway as well. Each quarterly is chock full of milestones and developmental phases.
We have a great news flow, growing revenues within the company, a solid and strategic board in place, USFDA approvals on the horizon.
Michael Frazis
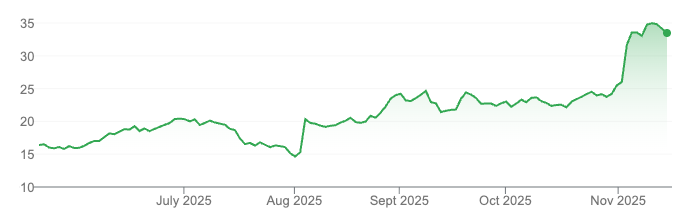
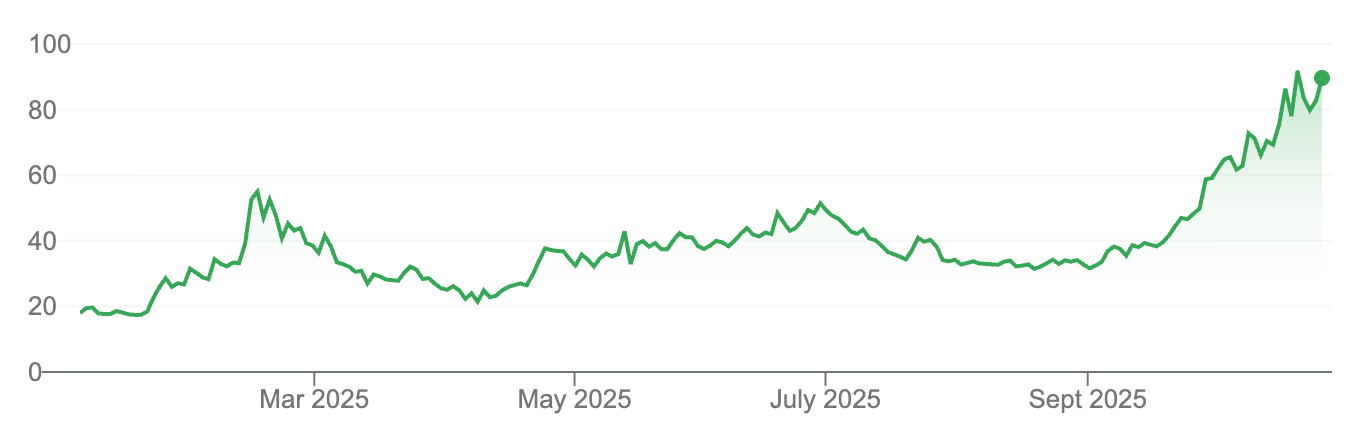
Absolutely. So the capital raise was at 60c?
Paul Anderson, Orthocell CEO
We did the cap raise at 60 cents and we raised $17 million, giving us a total of $35 million in the bank, which is a really strong position. We've seen great support on the market since then.
Michael Frazis
Thanks Paul, really appreciate it.
We obviously participated in that 60 cents cap raise and it's currently $1.10. So, it's been a good start for us.
Paul Anderson, Orthocell CEO
Happy days.
I'm in a really unique position, I get to interact with patients and I see the smile on their face and that's truly warms my heart every day.
I'm also able to work at the other end and provide opportunities for people to make money. Beyond just making money, my desire as a CEO is to have a fantastic business model.
To leave a legacy with Australian technology development and the manufacturing processes. We love that manufacturing side of it. We love the leverage that this product gives us in the market, and we're super excited about this next big phase of development.
Michael Frazis
Amazing, well so am I.
Thanks so much for taking the time to have a chat with us. Really appreciate it.
Paul Anderson, Orthocell CEO
My pleasure, Michael, and thanks so much for the support.
If you'd like to invest with us, you can access our investment portal and fund documentation through the button above.
Or simply reply to this email and we'll be in touch.
Disclaimer
The information in this note has been prepared and issued by Frazis Capital Partners Pty Ltd ABN 16 625 521 986 as a corporate authorised representative (CAR No. 1263393) of Frazis Capital Management Pty Ltd ABN 91 638 965 910 AFSL 521445. The Frazis Fund is open to wholesale investors only, as defined in the Corporations Act 2001 (Cth). The Company is not authorised to provide financial product advice to retail clients and information provided does not constitute financial product advice to retail clients.
The information provided is for general information purposes only, and does not take into account the personal circumstances or needs of investors. The Company and its directors or employees or associates will use their endeavours to ensure that the information is accurate as at the time of its publication. Notwithstanding this, the Company excludes any representation or warranty as to the accuracy, reliability, or completeness of the information contained on the company website and published documents.
The past results of the Company’s investment strategy do not necessarily guarantee the future performance or profitability of any investment strategies devised or suggested by the Company.
The Company, and its directors or employees or associates, do not guarantee the performance of any financial product or investment decision made in reliance of any material in this document. The Company does not accept any loss or liability which may be suffered by a reader of this document.